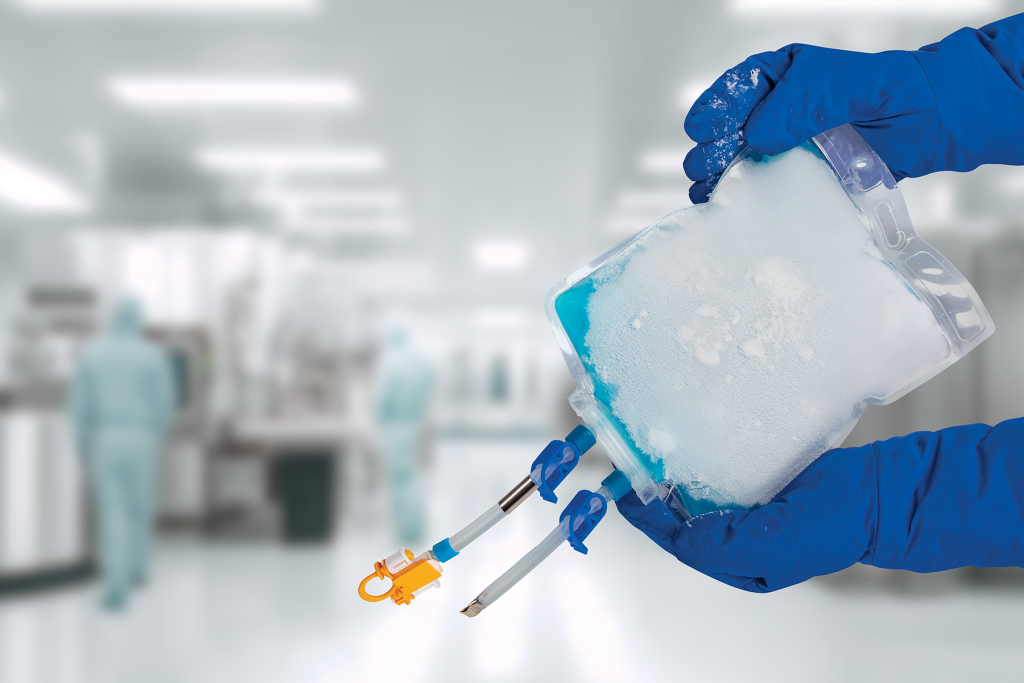
The robust security of the TepoFlex® polyethylene biocontainer film platform has been well established with clients over its nearly two decades of implementation. Leveraging the TepoFlex® film platform’s strengths, these biocontainers are manufactured to provide outstanding reliability and integrity throughout the harsh freeze/thaw cycle—extending the platform’s value for drug substance and drug product storage, transport, and process integration.
The biocontainer’s flexible single-use design can be easily integrated into existing freeze/thaw infrastructure. The biocontainer is compatible with a variety of freezers, including conventional blast freezers and vertical plate freezers. This enables deployment across various stages of drug development and manufacturing without major capital investment.
These freeze and thaw biocontainers are manufactured in ISO-certified cleanrooms with qualified componentry to ensure biocompatibility and support regulatory compliance. Meissner’s Applications Engineering Team offers consultation for the design and optimization of clients’ single-use systems, and can ensure assemblies are tailored to optimize application requirements.
To discover why leading biopharmaceutical manufacturers trust TepoFlex® for their most essential freeze and thaw applications, visit Meissner’s website at https://www.meissner.com/tepoflex-freeze-thaw where technical resources are available for download.
Additionally, visiting https://www.meissner.com/applications/freeze-thaw/ provides an overview of all of Meissner’s single-use freeze and thaw platform offerings.
Meissner’s manufacturing campuses include a headquarters location in Camarillo, California, USA, a European manufacturing location in Castlebar, Ireland, and buildout of a $250 million facility in Athens, Georgia.
Meissner’s product portfolios are built upon a solid foundation of quality, operational excellence, and technical expertise for delivery of high-performance products that support clients’ critical applications. For more information about Meissner, please visit https://www.meissner.com.